Tianda and Hebei University of Technology cooperate to make progress in cubic boron nitride synthesis
2024-06-08 03:06:34
Abstract Recently, the New Energy Materials Research Institute of Tianjin University and the Key Laboratory of Micro-Nano Boron Nitride Materials of Hebei University of Technology have closely cooperated to make important progress in the synthesis of cubic boron nitride. The research paper "photochemicalsynthesiso...
Recently, the Institute of New Energy Materials of Tianjin University and the Key Laboratory of Micro-Nano Boron Nitride Materials of Hebei University of Technology have worked closely together to make important progress in the synthesis of cubic boron nitride. The research paper "photochemical synthesis of ultrafine cubic boron nanoparticles "published in the top chemical journal "German Applied Chemistry" and was selected as a hot paper. 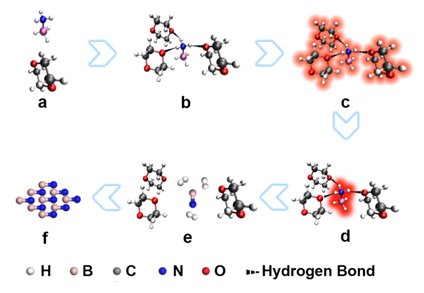
Du Xiwen Research Group of Tianjin University has made many pioneering achievements in the field of laser synthetic materials, and has taken the lead in realizing long-pulse-width laser controllable synthesis of nanomaterials. Tang Chengchun Research Group of Hebei University of Technology successfully realized the green synthesis, performance exploration and application development of new boron nitride based materials. Based on their respective advantages, the two parties cooperated in laser synthesis of cubic boron nitride research, tried a variety of process routes and material systems, and finally used laser irradiation of ammonia borane solution to synthesize ultrafine (3.5 nm) cubic nitrogen at normal temperature and pressure. Boron nanoparticles. It has been found that an aminoborane molecule can be combined with three polar solvent molecules to form a motif that can simultaneously absorb four laser photons and transfer energy to the aminoborane molecule, destroying its chemical bonds and completely removing it. hydrogen. Under the action of intense laser, a large amount of boron nitride molecules are generated in the solution, causing explosive nucleation of the boron nitride particles, thereby obtaining ultrafine boron nitride particles. The large radius of curvature of the ultrafine nanoparticles generates additional surface pressure, which makes the cubic phase stable at room temperature, and finally obtains cubic boron nitride particles.
The work achieved the following breakthroughs: 1) The synthesis of cubic boron nitride at room temperature and pressure was reported for the first time, and the synthesis speed was extremely fast, and the whole process only took 10 minutes. 2) For the first time, ultrafine cubic boron nitride particles were synthesized with a size of only 3.5 nm, and the hardness is expected to reach the highest value of the current material. 3) A one-step rapid and complete dehydrogenation of ammonia borane is realized, which provides a new process for rapid hydrogen release of hydrogen storage materials.
The study was supported by the National Natural Science Foundation.
H1 Led Headlight,H1 Headlight Bulb,H1 Headlight,H1 Led Light
CHANGZHOU CLD AUTO ELECTRICAL CO.,LTD , https://www.cld-leds.com