The Department of Chemistry won the research in the new low-cost non-precious metal electrolysis water catalyst
Hydrogen energy is an ideal energy carrier. The development of large-scale, cheap, clean, and efficient hydrogen production technology is the key to the effective use of hydrogen energy. Electrolyzed water has become one of the promising green hydrogen production methods due to its environmental friendliness, high product purity and no carbon emissions. The most important bottleneck restricting the large-scale application of electrolyzed water for hydrogen production is how to significantly reduce its electrical energy consumption, thus greatly reducing the cost of hydrogen production. The key is to develop low-cost, easy to prepare high-performance non-precious metal electrolyzed water catalyst, effectively reduce the overpotential of oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) on the electrode, and realize high current hydrogen production at low bath pressure.
With the support of the National Natural Science Foundation of China, the Ministry of Science and Technology and the Chinese Academy of Sciences, the Hu Jinsong research group of the Key Laboratory of Molecular Nanostructures and Nanotechnology, Institute of Chemistry, Chinese Academy of Sciences is committed to the design, controllable construction and catalytic mechanism of high-performance non-precious metal electrocatalysts the study. In recent years, they have designed and controlled high-intrinsic active sites of non-noble metal electrolyzed water catalysts, high-density, high-activity and effective catalytic sites, combined design of element-oriented reaction-oriented high active sites, and high-speed load and mass transfer. A series of progress has been made in the design of three-dimensional network structure electrodes (ACS Nano, 2016, 10, 851; J. Am. Chem. Soc., 2017, 139, 8320; Angew. Chem. Int. Ed., 2017, 56, 6572; Adv. Mater. 2017, 29, 1703311; Adv. Sci., 2017, 4, 1700084; Adv. Energy Mater. 2018, 8, 1800734; Adv. Energy Mater. 2018, 8, 1801698; Adv. Funct. Mater . 2018, 28, 1704594; Small Methods 2019, 3, 1800317). Recently, they have developed a new in-situ electrochemical conversion strategy, through in-situ non-metallic Se doping to achieve a low-cost, high-performance iron-based oxygen evolution water electrolysis catalyst, the relevant results published in J. Am. Chem. Soc., 2019, 141, 7005, and was selected as the cover of the magazine.
Nickel and cobalt are usually indispensable components of high-performance OER catalysts due to the proper adsorption and desorption ability of intermediate species with oxygen evolution reaction. Although iron is much more abundant and cheaper than nickel and cobalt, the current performance of iron-based catalysts for oxygen evolution is far lower than nickel and cobalt-based materials. The researchers first used theoretical calculations to reveal that non-metallic Se doping can significantly improve the rate of electrochemical oxygen evolution in all-iron-based FeOOH catalysts. Based on this, a simple in situ electrochemical activation strategy was developed. In situ electrochemical activation of FeSe vertical nanosheet arrays grown on a three-dimensional conductive low-cost foamed iron substrate under OER conditions was converted to Se-doped by in-situ electrochemical activation The FeOOH nanosheet array enables the all-iron-based catalyst to have both high intrinsic active sites and high electrochemically active area, so it exhibits OER electrocatalytic activity equivalent to that of all-nickel or all-cobalt catalysts. This strategy is also applicable to the preparation of a small amount of nickel-doped iron-rich catalyst, so that a small amount of nickel (3.3 at%) doped iron-rich catalyst can achieve electrocatalytic OER performance comparable to the best nickel-rich or cobalt-rich catalyst. The overpotential at a current density of 500 mA cm-2 requires only 348 mV and has excellent stability. When the catalyst is applied to an electrolyzed water device, the tank pressure at a current density of 50 mA cm-2 requires only 1.62V. In an actual sunlight-driven water splitting system, the catalyst achieves one of the highest conversion efficiencies of 18.55% of sunlight to hydrogen. These results indicate that the strategy provides a possible way to develop new, abundant, economical, and efficient electrolytic hydropower catalysts.
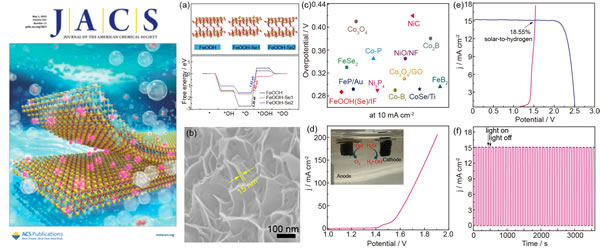
In-situ conversion of Se doping strategy to construct a low-cost, high-efficiency iron-based oxygen evolution catalyst
Ultrasonic Flowmeter,Portable Flow Meter,Portable Ultrasonic Flow Meter,Ultrasonic Water Flow Meter
Wuxi Winsun Automation Instrument Co., Ltd , https://www.winsunwx.com