Preparation and surface analysis of bionic superhydrophobic cotton fabric
Wang Qiang, An Qiufeng, Liu Yue, Li Xianqi, Yuan Junmin (Key Laboratory of Light Chemical Additive Chemistry and Technology, Ministry of Education, College of Chemistry and Chemical Engineering, Shaanxi University of Science and Technology, Xi'an 710021, China)
Abstract: Using the micro/nano structure of the lotus leaf surface as a reference model, the natural cotton fabric was treated with silica sol first, followed by N-β-aminoethyl-γ-aminopropyl polydimethylsiloxane (ASO-1). It was modified to obtain a micro/nano binary rough superhydrophobic fabric, and the contact angle of water droplets on the surface of the fabric was up to 160.5°. Field emission scanning electron microscopy (FE-SEM) showed that a large number of uniformly distributed nano-microprotrusions existed on the surface of superhydrophobic fibers. The contact angle analysis indicates that the micron-scale roughness and the presence of ASO-1 film formed during the weaving process of the fabric are the main reasons for the hydrophobicity of the fabric. The nano-protrusion can reduce the contact area of ​​the fiber with water and increase the contact angle of water on the fiber surface. The fabric changes from hydrophobic to superhydrophobic. Finally, the presence of SiO2 particles and ASO-1 film on the fiber surface was confirmed by X-ray photoelectron spectroscopy (XPS).
Key words: superhydrophobic; cotton fiber; aminosilicon; silica sol CLC number: TB39 Document code: A Article ID:1000-7555(2010)04-0160-03
Superhydrophobic means that the static contact angle of solid surface to water is 150° or higher. Whether superhydrophobicity can be achieved is determined by the combination of chemical composition and microstructure of solid surface [1, 2]. There are many examples of superhydrophobic surfaces in nature, such as lotus leaf [3], rice leaf [4] and so on. Super-hydrophobic fabric can be used in rain/snowwear, tents, military combat uniforms, etc. Its huge potential market and economic benefits make it a hot spot for superhydrophobic interface research. Generally, superhydrophobic fabrics can be obtained by modifying the roughened fibers with small molecules [5, 6], but the small molecule modification method takes a long time and it is difficult to form a dense hydrophobic membrane. Amino-modified polydimethylsiloxane has low surface tension and good film-forming properties, and is specially arranged on the surface of the fabric [7], and is expected to be a low-energy substance for constructing super-hydrophobic fabrics. Inspired by the surface structure of the lotus leaf, the author used N-β-aminoethyl-γ-aminopropyl polydimethylsiloxane as a low-energy substance combined with gel sol method to treat the fiber surface, and obtained a contact angle of 160.5°. Hydrophobic fabrics were studied and their surface chemical composition, morphology and hydrophobicity were studied.
1 Experimental part 1.1 Raw materials and reagents Cotton cloth: 100% cotton, industrial products, washed with acetone before use; silica sol (30% (mass fraction, the same below), pH=9), industrial products, Qingdao Hengshengda Chemical Co., Ltd. Company; toluene, analytical grade, Tianjin Dengfeng Chemical Reagent Factory; N-β-aminoethyl-γ-aminopropyl polydimethylsiloxane (ASO-1, Fig.1): self-made, ammonia value 0 · 5905 mmol / g, weight average molecular weight - Mw = 37,500, number average molecular weight - Mn = 14100 (measured by WaterGPC, the reference is polystyrene, the solvent is tetrahydrofuran). 
1.2 Preparation of super-hydrophobic cotton fabric Preparation of super-hydrophobic fabric: Take appropriate amount of silica sol in a beaker, dilute with distilled water to a solid content of 3%, adjust pH to 5~6 with hydrochloric acid, and cut to length × width. A 2 cm × 1 cm cotton cloth sample was immersed in the diluted silica sol for several seconds, taken out, and baked in an oven at 100 ° C for 10 min. It was further immersed in a dilute solution of 0.25% ASO-1 in toluene for 1 h, and dried in an oven at 100 °C.
Preparation of reference hydrophobic fabric: The same swatch was immersed in a dilute solution of 0.25% ASO-1 in the same way.
1.3 Characterization 1.3.1 Chemical composition of the sample surface: Analytical with a British Axis Ultra photoelectron spectrometer (Kratos Analytical Ltd., UK). The X-ray source is monochromatic Al Kα ray, angular resolution 90°, chamber vacuum: 1 · 2 × 10-8 Pa, the binding energy deviation caused by the charging effect is corrected by the carbon C1s peak (284·8 eV) of the sample surface contamination.
1.3.2 Observation of surface morphology: The sample was vacuum-sprayed and observed and photographed by FEI SEIION 200 FE-SEM.
1.3.3 Sample hydrophobicity test: Characterized by static contact angle of water on the surface of the sample, measured by Shanghai Zhongchen Digital Equipment Co., Ltd. JC2000C1 contact angle measuring instrument, each sample was measured 5 times and averaged.
2. Results and discussion 2.1 Surface morphology observation Fig. 2 is an FE-SEM image of the surface morphology of the fiber before and after modification. It can be seen from Fig. 2a that the cotton fabric has a binary roughness on the micrometer scale after weaving, and the weaving method of the yarn (the diameter of about 150 μm~200 μm) is the structural unit, so that the cotton fabric has a large micrometer scale. With a certain roughness, the arrangement of a plurality of fiber bundles (diameter of about 10 μm to 20 μm) per yarn allows the yarn to exhibit a certain roughness on a small micrometer scale. It was observed that the single natural cotton fiber bundle was not very flat under a magnification of 5000 times, and further enlargement observed that the fiber bundle itself had a certain roughness at the nanometer scale. The FE-SEM of the modified fiber is shown in Fig. 2(d,e,f). It can be found from the FE-SEM photograph before the modification that the morphology of the treated fabric on the micrometer scale hardly changes, and still has a large Roughness. The surface of a single fiber bundle is very rough, and there are many gullies and particles. Under the condition of 50,000 times magnification, it can be observed that the surface of the fiber is not only uneven, but also a large number of nano-protrusions having a diameter of about 30 nm exist. 
2.2 Hydrophobicity analysis Fig. 3a is a photograph of the contact angle of the surface of the untreated cotton fabric. It can be seen that the surface of the natural cotton fabric is very hydrophilic, and the water droplets can be absorbed quickly on the surface, so the contact angle is 0°. After ASO-1 treatment, ASO-1 can form a low-energy hydrophobic membrane on the surface of the fiber with silicon methyl toward air and silicon-oxygen dipole pointing to the fiber interface [7], so the fiber becomes hydrophobic and its static The contact angle is 148° (Fig. 3c). The static contact angle of the fabric surface treated with silica sol and then modified with ASO-1 is 160.5° (Fig. 3d), which indicates that the formation of nano-protrusions is beneficial to improve the hydrophobicity of the fabric surface and enable the fabric to be Hydrophobic becomes superhydrophobic. 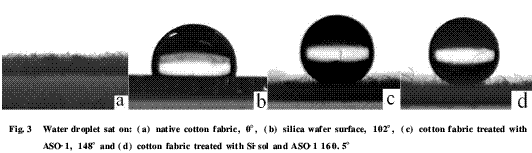
The change of the contact angle of the fabric surface before and after the modification can be found that the roughness of the cotton fabric on the micrometer scale is the main reason for the increase of the hydrophobicity of the fabric, so the contact angle of the water droplet on the surface of the ASO-1 treated fabric is much larger than that. Flatten the contact angle of the substrate surface (102°, see Fig. 3b). The formation of nano-micro-convex on the fiber surface contributes to the increase of the contact angle value, which indicates that the action of the micro-protrusion is likely to be similar to that produced by the nanostructure above the surface of the lotus leaf, that is, the formation of the nano-protrusion It helps to absorb more air when the water droplets are in contact with the fiber, reduces the contact area between the fabric and the water, and finally changes the wetting type of the fabric to water by the Cassie adsorption model to the Wenzel adsorption model. The role of nano-micro-convex in the formation of super-hydrophobic fabrics can be described by the Cassie-Baxter formula:
Cosθ*=Φs(cosθ+1)-1 (1)
Where: θ*—the contact angle of water on a rough surface; θ—the contact angle of water on a smooth surface (102°); Φs—the interface of fabric-water at the fabric-water and air-water interface Area score. It can be estimated from the formula that the surface Φs of the fabric modified only by ASO-1 is 19.2%, and the surface Φs of the fabric with nanoprotrusions is reduced by 7.2%. The calculation results show that the presence of the nano-protrusions allows more air to be wrapped between the fabric and the water droplets, which reduces the water-fiber contact area fraction, prevents the fabric from being wetted by water, and the super-hydrophobic fabric is constructed.
2.3 Analysis of surface chemical composition The chemical composition of the surface of the fabric before and after modification was analyzed by XPS, see Fig. 4. It can be seen from the figure that only 285 eV and 531 eV absorption peaks appear on the surface of natural cotton fabric, which are attributed to O1s and C1s, respectively, and the content of each element is O: 38.45%; C: 61.55%, which is analyzed with the literature [8]. The results are similar and are also close to the theoretical element content of cellulose. The appearance of Si2s absorption peak at Si2p and 150 eV at 102 eV on the surface of fabric treated with silica sol indicates that SiO2 particles exist on the surface of the fiber bundle after treatment, and the absorption peak of C1s is obviously reduced. The content of C element is only 14.53%, which further explains the treatment. There are a large amount of SiO2 particles on the surface of the cotton fabric.
In contrast, the XPS spectrum of the fabric modified by ASO-1 not only showed the absorption peaks belonging to O1s, C1s, Si2p, Si2s, but also the high resolution spectrum of N1s absorption peak and C element content increased at 399 eV. A 39.92% can prove the presence of ASO-1 film on the surface of the fiber. 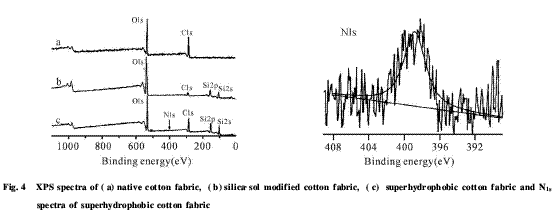
3. Conclusions The natural cotton fabric was modified with ASO-1 and silica sol to obtain a micro/nano binary rough superhydrophobic cotton fabric with a water contact angle of 160.5°. The micron-scale roughness produced by the fabric during spinning and weaving and the formation of a hydrophobic ASO-1 film are the main reasons for the increased hydrophobicity of the fabric. The formation of SiO2 nano-protrusions can reduce the fabric-water contact area, prevent the water from wetting the fabric, increase the contact angle of water on the surface of the fabric, and transform the fabric from hydrophobic to super-hydrophobic.
References: slightly
Hand Pump Led Light And Safety Tools
Hand Pump,Foot Pump,Oil Can,Grease Gun
SHAOXING SINO IMPORT & EXPORT CO.,LTD , https://www.sxsmarto.com